C o n t e n t s
Abstract
1. Introduction 1
1.1 Role of Ozone in the Lower Atmosphere 1
1.2 Uncertain Issues of Ozone Depletion Events 1
1.3 Role of NOx in Ozone Depletion Events 3
2. Model and Methods 4
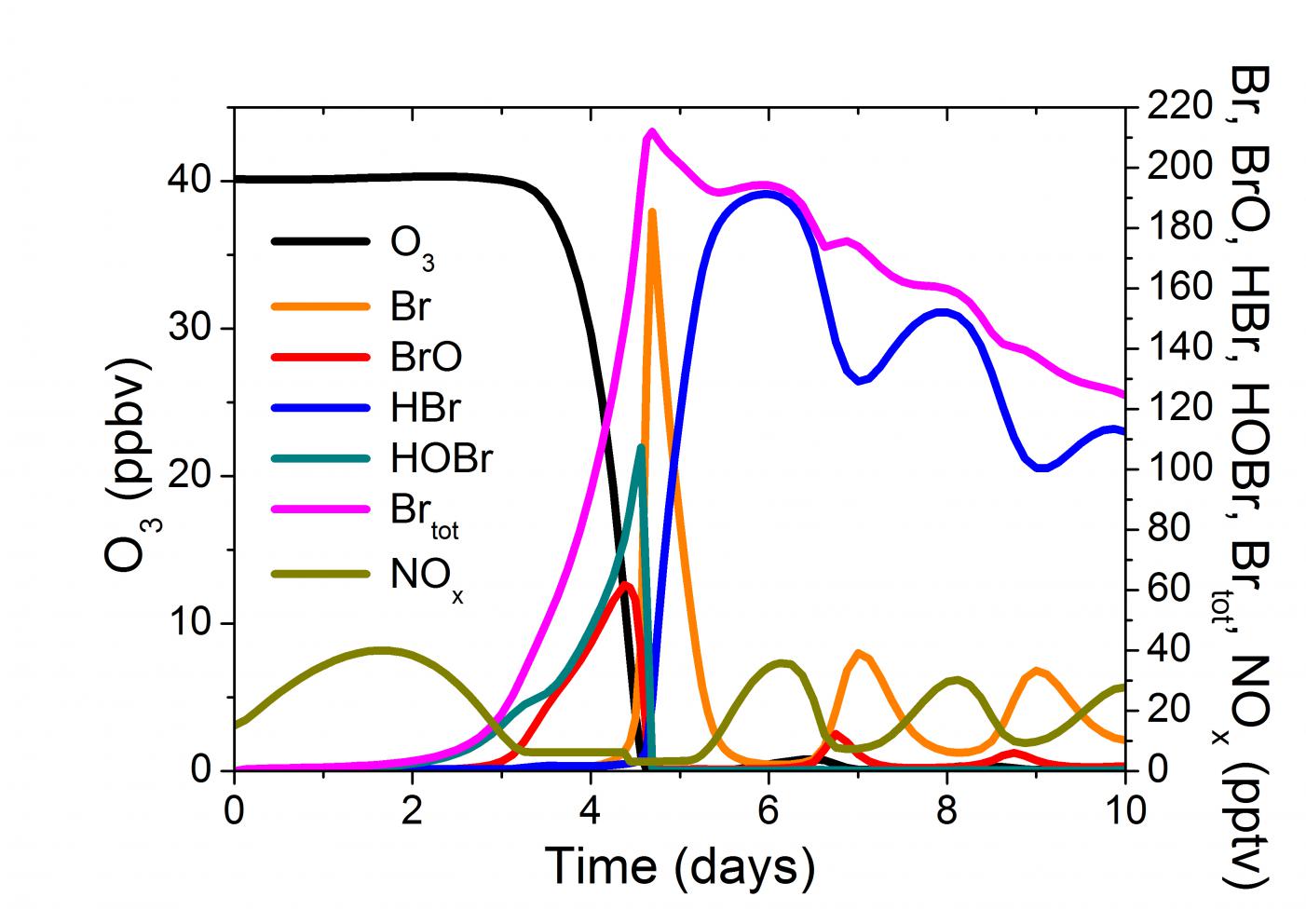
3. Results and Discussions 7
3.1 Standard Case 7
3.2 Modification of the NOx Emission 8
3.3 Modification of the NOx Initial Concentration 11
4. Conclusions 14
References 16
Acknowledgements 19
A model study: role of the nitrogen oxides in the ozone depletion events during the springtime of Arctic
Liting Liu
School of Atmospheric Physics,NUIST,Nanjing 210044,China
Abstract: By adopting a model containing 39 chemical species and 92 related reactions, this research investigates the role of the nitrogen oxides (NOx) in the ozone depletion events (ODEs) during the springtime of Arctic. We simulated three scenarios in the present study. First, a typical atmospheric composition in Arctic was applied in the model to capture the temporal variation of ozone and corresponding bromine containing species as a standard case to be compared with subsequent cases. Second, while the initial NOx concentration of 15 pptv was kept the same, the NOx emission flux rate was modified to study the effect of NOx emission from the underlying surface on the ozone depletion and halogen liberation. At last, under the condition of an unchanged emission rate in the model, the initial concentration of NOx is changed to different values to investigate how the nitrogen oxides influence the evolution of ozone during ODEs. It can be inferred from the results that in the latter two cases, the NOx emission rate and initial NOx concentration both have a turning point. That is to say, the effect of NOx on ozone depletion is not monotonically positive or negative correlation, but has a critical value or a range of value. Therefore, it is concluded that when the NOx concentration is relatively low close to the background level (~40ppbv), the ozone depletion events are accelerated due to the elevated concentration of halogen species. On the contrary, if the NOx concentration is significantly higher than the background value, the ozone depletion beginning time is delayed.
Key words: ozone depletion; nitrogen oxides; Arctic; initial concentration; emission
- Introduction
- Role of Ozone in the Lower Atmosphere
Ozone is a kind of trace gas of great significance in the atmosphere, which has a great impact on the global climate and the environment. Most of the ozone is concentrated in the stratosphere, about 10 to 50 km, while the content of ozone in the troposphere accounts for approximately 10% of the total ozone[1]. Ozone is the key composition of the atmospheric dynamic, thermodynamic, radiant and chemical processes in both stratosphere and troposphere, playing a vital role in the climate and environmental change. First of all, ozone is a strong oxidant, whose distribution and variation directly affect the concentration and lifetime of other chemical species and free radicals such as OH. Secondly, by absorbing the Ultra-Violet (UV) radiations from the solar radiation, ozone becomes the main heat source in the stratosphere and protects the life in the troposphere from the harm of UV radiations. Thus, to a large extent, the existence of ozone determines the temperature structure of stratosphere. Moreover, having a strong absorption band at 9.6 μm, ozone is an important greenhouse gas. It has a cooling effect in the middle and upper part of stratosphere, while in the low level of the stratosphere and the troposphere, it has a warming effect[2]. Most of the ozone in the atmosphere originates from ionization of oxygen through lightning, but with the increase of the anthropogenic emissions such as NOx and VOCs which are precursors of ozone, the mixing ratio of ozone has doubled since 1900s[3]. Although few people reside in Arctic, precursors derived from human emissions in ambient cities may be transported to Arctic region. Thus, it is considered that the content of ozone in Arctic is more or less influenced by anthropogenic activities.
- Uncertain Issues of Ozone Depletion Events
Investigations on ozone depletion phenomenon are always the central issues in many fields. The stratosphere and troposphere both have the different type of depletion, in which the Antarctic ozone hole discovered by Farman et al.[4] is the most well-known phenomenon occurring in the stratosphere. In this study, we focus on the ozone depletion events in troposphere. In 1978 in Barrow, Alaska, it was observed by Oltmans et al.[5] that the amount of ozone in the Arctic boundary layer dropped rapidly from background level (~40ppb) to less than 1ppb within several days during springtime, which was called ozone depletion events (ODEs). Furthermore, the low concentration of ozone lasted for a few days until the local meteorological condition had an obvious variation. Afterwards, Bottenheim et al.[6] also observed the analogous phenomenon in the troposphere in Alert, Canada in 1986 and confirmed the discovery. Correspondingly, the concentrations of halogen species, especially the bromine containing species increased apparently during the ODEs[7]. That is to say, the depletion of ozone has a close relationship with the increase of the bromine containing species. In fact, bromine species have a chemical reaction cycle as following:
Br2 hν → 2Br
剩余内容已隐藏,请支付后下载全文,论文总字数:50498字
相关图片展示:
该课题毕业论文、开题报告、外文翻译、程序设计、图纸设计等资料可联系客服协助查找;