炔酰胺的顺式胺硼化反应研究毕业论文
2020-06-07 21:13:36
摘 要
一个有效的广泛使用的试用的过程是报道的C-H键在杂环直接烷基化,在许多科学领域享有特权的支架。这种反应是基于铜催化活性的二级和三级烷基溴化物广泛的芳烃,包括呋喃、噻吩、吡咯烷基自由基的产生,和苯并衍生物,以及香豆素累和喹诺酮类。
在令人印象深刻和不断增长的化学反应,芳烃的烷基化显然是最重要的,由于选择性烷基化芳烃为主要原料,大多数科学领域,其余有一个小的有机分子所需要的相关分子,它们确实由于制药、农业化学和材料科学的日常工作,并作为高分子化学或有机电子的基石。他们也对人类健康和许多其他部门产生了深远的影响。
芳烃的选择性烷基化反应是通过德高望重的弗里德尔-Crafts反应传统的表演,一个反应所需要的的反应条件苛刻,经常遭受化疗和/或区域选择性。这种反应也阻碍了显着的限制,在电子性质的芳烃,他表现不佳,与某些类别的卤代烷。芳烃的烷基化反应的主要替代策略太多基于化学计量反应金属化后的亲电试剂2-a序列受到限制的官能团兼容性由于要求强基础或逐步预修饰后金属介导的交叉耦合。
在过渡金属催化的C-H官能化的最新进展已使非常有效的和可靠地芳烃的直接烷基化工具的发展,现在出现了强大的替代品的经典过程。最常见的策略来保证高水平的反应活性和选择性主要是基于介绍,这将允许一个单一的C-H键启动芳烃选择性烷基化。随着穆雷对芳基酮提供邻位烷基化的开创性工作性,一个系列的烷化剂进行直接的邻位,芳烃烷基化更是很少。互补的策略,已规避指导组的试用,是基于杂环化合物C-H的内在化。一个有效的试剂和程序已经被设计为选择性引入烷基取代基为多种杂环的方法,主要是缺电子具有酸性的C-H键唑。形成鲜明对比的是,以支付其他杂环的方法,直接烷基化。注意,尽管这样的反应强烈的合成电位少得多。
关键词:炔酰胺 碘氟化
Abstract
An efficient and broadly applicable process is reported for the direct alkylation of C–H bonds in heteroarenes, privileged scaffolds in many areas of science. This reaction is based on the copper-catalyzed addition of alkyl radicals generated from activated secondary and tertiary alkyl bromides to a wide range of arenes, including furans, thiophenes, pyrroles, and their benzo-fused derivatives, as well as coumarins and quinolinones.
Among the impressive and ever-growing number of chemical transformations, the alkylation of arenes is clearly of prime importance, due to regioselectively alkylated arenes being major starting materials and molecules relevant to most areas of science in which there is a need for small organic molecules. They are indeed used on a daily basis in the pharmaceutical, agrochemical and material sciences, and as building blocks for polymer chemistry or organic electronics. They also have had a profound effect in human health and many other sectors.
The regioselective alkylation of arenes is traditionally performed through the venerable Friedel–Crafts reaction,1a reaction which requires harsh reaction conditions and often suffers from low chemo- and/or regio-selectivities. This reaction is also hampered by significant limitations in terms of the electronic properties of the arene, and it performs poorly with some classes of alkyl halides. The main alternative strategies for the alkylation of arenes are mostly based on stoichiometric metallation followed by reaction with an electrophile2—a sequence which suffers from limitations in terms of the functional group compatibility due to the requirement for strong bases—or on stepwisepre-functionalization followed by metal-mediated cross-coupling
Recent advances in transition-metal catalyzed C–H functionalization have enabled the development of remarkably efficient and reliable tools for the direct alkylation of arenes, which now appear as robust alternatives to the classical processes. The most common strategy to ensure high levels of reactivity and selectivity is mostly based on the introduction of a directing group, which will allow for the selective alkylation of a single C–H bond of the starting arene. Following the pioneering work of Murai on the ortho-selective alkylation of arylketones, a range of directing groups and alkylating agents have been introduced for the direct ortho- and, much more rarely, meta-alkylation of arenes. A complementary strategy, which circumvents the use of directing groups, is based on the innate alkylation of C–H bonds in heteroarenes. A number of efficient reagents and procedures have indeed been designed for the regioselective introduction of alkyl substituents into a variety of heteroarenes, mostly electron-deficient ones and azoles possessing acidic C–H bonds. In sharp contrast, much less attention has been paid to the direct alkylation of other heteroarenes, despite the strong synthetic potential of such reactions
KEY WORDS:ynamides iodoflurination
目录
摘要 2
Abstract 3
第一章 文献综述 1
1.1 前言 1
1.2 酰胺的性质 1
1.3炔酰胺碘氟化的研究意义 1
第二章 实验 3
2.1前言 3
2.2二氟甲烷与炔反应 3
2.3 实验过程及数据 3
第三章 结论与总结 6
4.1 前言 6
4.2 总结 6
第四章 参考文献 7
致谢 8
附录 核磁谱图 9
E-2a,C谱 9
E-2a,F谱 10
E-2a,H谱 11
E-2b,C谱 12
E-2b,F谱 13
E-2b,H谱 14
第一章 文献综述
1.1 前言
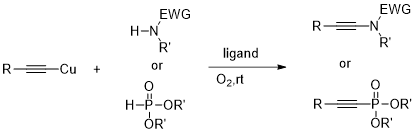 氮-磷基亲核试剂可以顺利转化为相应的杂原子取代的炔烃在室温下简单反应和铜乙炔氧气气氛下。这些稳定的、现成的,聚合物作为特别有效的药剂对炔基团的引入显著轻微的氧化条件,允许他们在正式的极性转换。螯合配体如甲基苯胺和N-甲基咪唑是有效的有机助剂,即使复杂的基板,能使他们清洁和快速氧化的交叉耦合。
氮-磷基亲核试剂可以顺利转化为相应的杂原子取代的炔烃在室温下简单反应和铜乙炔氧气气氛下。这些稳定的、现成的,聚合物作为特别有效的药剂对炔基团的引入显著轻微的氧化条件,允许他们在正式的极性转换。螯合配体如甲基苯胺和N-甲基咪唑是有效的有机助剂,即使复杂的基板,能使他们清洁和快速氧化的交叉耦合。
1.2 酰胺的性质
羧酸中的羟基被氨基(或胺基)取代而生成的化合物,也可看成是氨(或胺)的氢被酰基取代的衍生物。酰胺中氨基上的氢原子可形成氢键,发生分子间的缔合,使酰胺的沸点比相应的羧酸高。酰胺广泛分布于自然界,蛋白质是以酰胺键─CONH─(或称肽键)相连的天然高分子化合物。酰胺在强酸强碱存在下长时间加热,可水解成羧酸和氨(或胺)。酰胺可以通过羧酸铵盐的部分失水或从酰卤、酸酐、酯的氨解来制取;腈也可部分水解,停止在酰胺阶段。
1.3炔酰胺碘氟化的研究意义
最近,我们发现碘分子作为强大的亲电试剂当允许在不同碘(III)物种存在下与不饱和有机化合物反应。这种化学的一个例子是一个新的方便区域和立体选择性反应的苯烯烃和炔烃的促进4-二氟化碘甲苯和二笨二硒。这两种试剂4-tolif2经历了个非常快速和有效的氧化以及碘之间的任何竞争反应(III)物种和不饱和有机化合物避免。
现在,我们注意力也应该引起关在毒性更小,更环保的碘与4-碘甲苯氟化反应存在双重或三重的C-C键。在各种不饱和烃研究了几种反应条件,在文献中描述的一些方法和利用非常难处理的试剂如元素氟,氟化钾(氟化氢)盐、或二氟化氙在碘的存在或者NIS的存在下。其他相关方法用双(吡啶)碘鎓四氟硼酸盐在低温四氟硼酸的存在,一个更多的最近的离子液体emimf和氟化氢混合。电化学生成的碘鎓阳离子是有用的生产几种fluoroiodoalkanes都和一个fluoroiodoalkene在普通的特征。所有的这些合成方法是他们选择使用性的形成的研究fluoroiodoalkanes而得到的是fluoroiodoalkenes好基本上从末端炔烃好使用一种两种的方法。
课题毕业论文、开题报告、任务书、外文翻译、程序设计、图纸设计等资料可联系客服协助查找。



