钨基化合物用于锂离子电池负极材料毕业论文
2020-06-17 21:33:09
摘 要
最近几年,在锂离子电池负极材料研究中,钼基材料是大家研究的比较深入的材料,它属于理论容量较高的过渡金属负极材料。钨与钼同属ⅥB一族,最外层电子数相同,电化学性质相似,本论文主要研究钨基氮化物应用于锂离子电池负极材料的电化学性能。由于商业氮化钨颗粒较大,导致转化反应无法顺利进行,实际比容量低;且在长时间的充放电过程中体积效应明显,会对其结构造成不可逆破坏,从而造成循环稳定性差。碳是一种良好的导电材料,将其与纳米氮化钨材料复合不仅可以增强其导电性,还能对Li离子嵌入和脱出过程中的体积变化起到缓冲作用,从而增加氮化钨材料的循环稳定性。本论文的主要研究内容是通过制备多孔碳/钨基氮化物复合材料来提高氮化物作为负极材料时的实际比容量
本论文结合离子交换法和熔盐法制备了不同钨离子吸附浓度的多孔碳/氮化钨纳米复合材料,然后分别进行X射线衍射测试(XRD)、X射线光电子能谱分析测试(XPS)、扫描电子显微镜测试(SEM)、氮气吸脱附测试。并组装成纽扣式半电池,对其进行循环伏安、恒电流充放电等电化学性能进行测试。通过对比发现钨离子吸附浓度为0.3 mol L-1时,所得负极材料性能最优。在电流密度为100 mA g-1时,循环50圈后材料比容量能稳定保持在544.3 mA h g-1,体现了良好的循环稳定性。且在2000 mA g-1的高倍率电流密度下,材料仍能保持222.8 mA h g-1的比容量,体现了优越的倍率性能。相比于商业的氮化钨,本文所得的多孔碳/氮化钨用作锂离子电池负极材料时性能有了很大提高。主要原因是多孔碳增强了材料的导电性,氮化钨的纳米颗粒缩短了Li离子的传输距离,容量得到提升。复合材料的多孔性质使得材料与电解液的接触面积增大,有利于Li离子脱嵌,且脱嵌过程中的体积膨胀得以适当缓解,提高了材料的循环稳定性。
关键词:锂离子电池 负极材料 氮化钨 熔盐法 多孔碳
Tungsten-based composites as the anode materials of lithium-ion batteries
ABSTRACT
In recent years, molybdenum-based materials as the anode materials of lithium-ion batteries have attracted great research attention for their large theoretical capacity. Tungsten belongs to the same ⅥB family as molybdenum, thus having the same outermost electrons and similar electrochemical properties. Therefore, here we have focused on tungsten nitride as the anode material of lithium-ion batteries. It is difficult to meet the needs of large capacity in modern society, there is a large practical potential that the tungsten nitride material can make it because of its high theoretical capacity. Actually, it is extremely hard to obtain a high actual capacity. The main challenge of this paper is to improve the specific capacity of the tungsten nitride by compositing it with porous carbon. However, the volume effect is serious during the long charging and discharging process, which results in irreversible damage to its structure and poor cycling stability. Carbon is so excellent a conductive material that we can enhance the conductivity by combining it with nanostructured tungsten nitride m, and the volume change can be buffered during the Li-ion insertion/extraction process, thus increasing the cycling stability of the material.
In this paper, we combined the ion-exchange method and the molten-salt method to prepare tungsten nitride/porous carbon nanocomposite. X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS) and nitrogen adsorption-desorption were used to characterize the materials. Electrochemical properties were tested by the cyclic voltammograms test and the constant current charge/discharge test after the half cells were assembled. We found that the tungsten ion adsorption concentration of 0.3 mol L-1 was optimal in comparison with others, which had excellent performance. The specific capacity stabilized at 544.3 mA h g-1 at the current density of 100 mA g-1 after 50 cycles, which
indicated good cycling stability. The specific capacity remained 222.8 mA h g-1 at the current density of 2000 mA g-1, suggesting its high rate performance. Compared with the commercial tungsten nitride, the performance of the porous carbon/tungsten nitride composite improved a lot. The main reason is that the porous carbon enhances the conductivity of the material and the nanoparticles of the tungsten nitride shorten the transmission distance of Li ions, which cause the improved specific capacity. The porous properties of the composites increase the contact area between the material and the electrolyte, which is favorable for Li ion insertion and extraction, and the volume expansion in the process of insertion and extraction can be appropriately alleviated and the cyclic stability of the material is improved.
Keywords: Lithium-ion battery; Anode material; Tungsten nitride; Molten-salt method; porous carbon
目 录
摘 要 I
ABSTRACT II
第一章 绪论 1
1.1 引言 1
1.2 锂离子电池原理 1
1.3 锂离子电池的特点 3
1.4 锂离子电池负极材料 4
1.4.1 碳负极材料 4
1.4.2 合金类材料 4
1.4.3 过渡金属氧化物材料 4
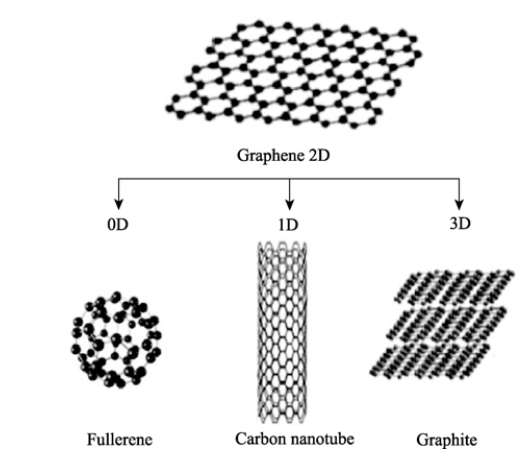
1.4.4 石墨烯负极材料 4
1.5 氮化钨负极材料 5
1.6 本论文的研究内容 6
第二章 实验部分 7
2.1 试剂及仪器 7
2.2 电池制备 8
2.2.1 活性物质制备 8
2.2.2 电池壳的处理 8
2.2.3 隔膜的制备 9
2.2.4 泡沫镍片的制备 9
2.2.5 浆料的调配 9
2.2.6 组装纽扣电池 9
2.3 材料表征 10
2.3.1 X射线衍射 10
2.3.2 扫描电子显微镜 10
2.3.3 BET测试 10
2.3.4 XPS测试 11
2.4 电化学性能测试 11
2.4.1 恒流充放电测试 11
2.4.5 循环伏安测试 11
第三章 结果与讨论 12
3.1 物理表征 12
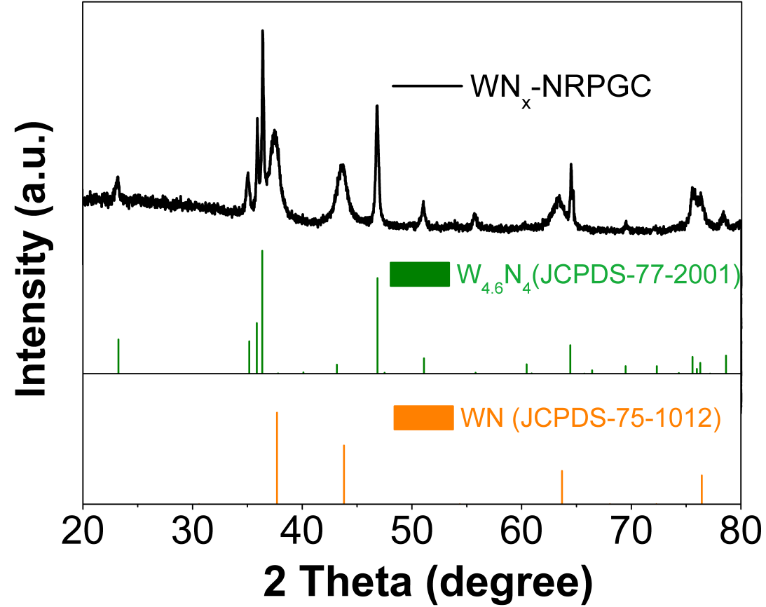
3.1.1 XRD分析 12
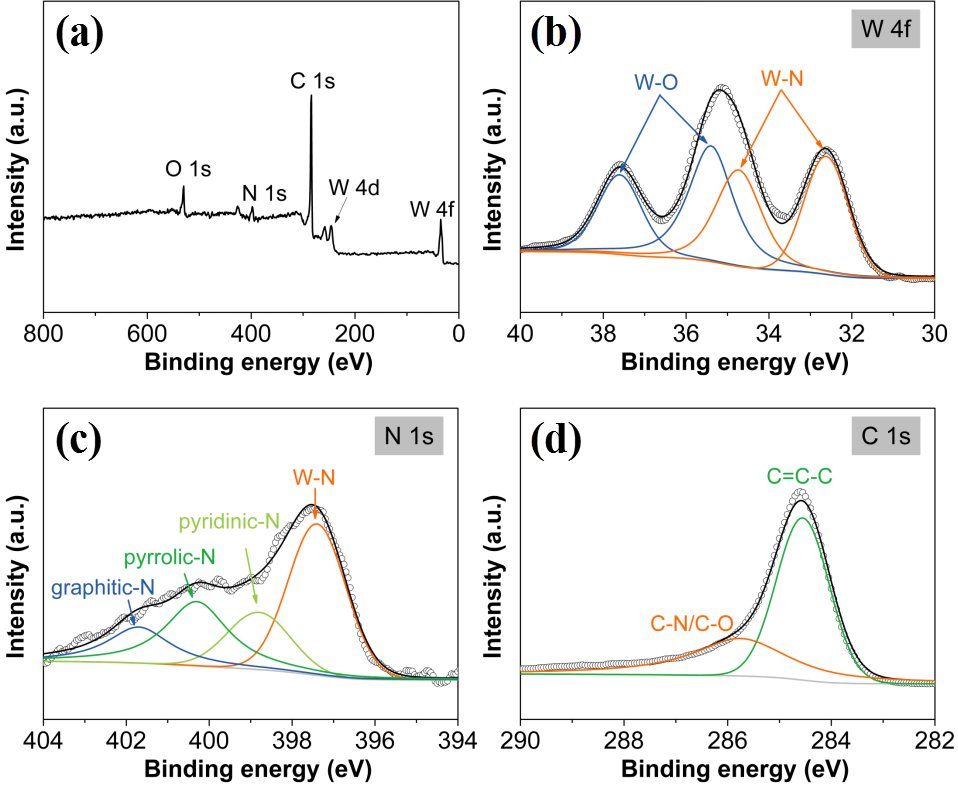
3.1.2 XPS分析 12
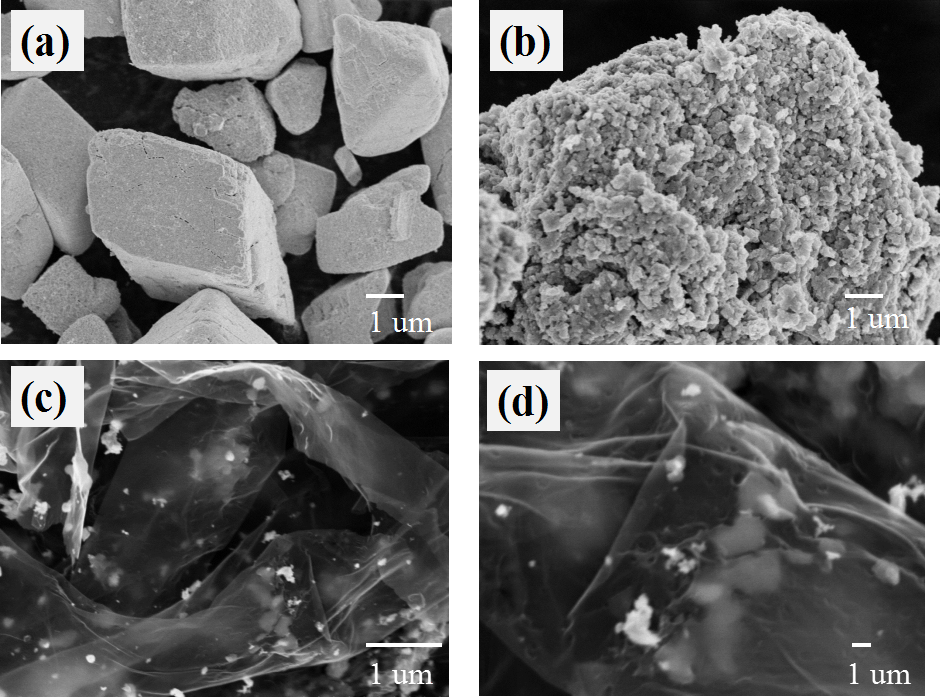
3.1.3 SEM分析 13
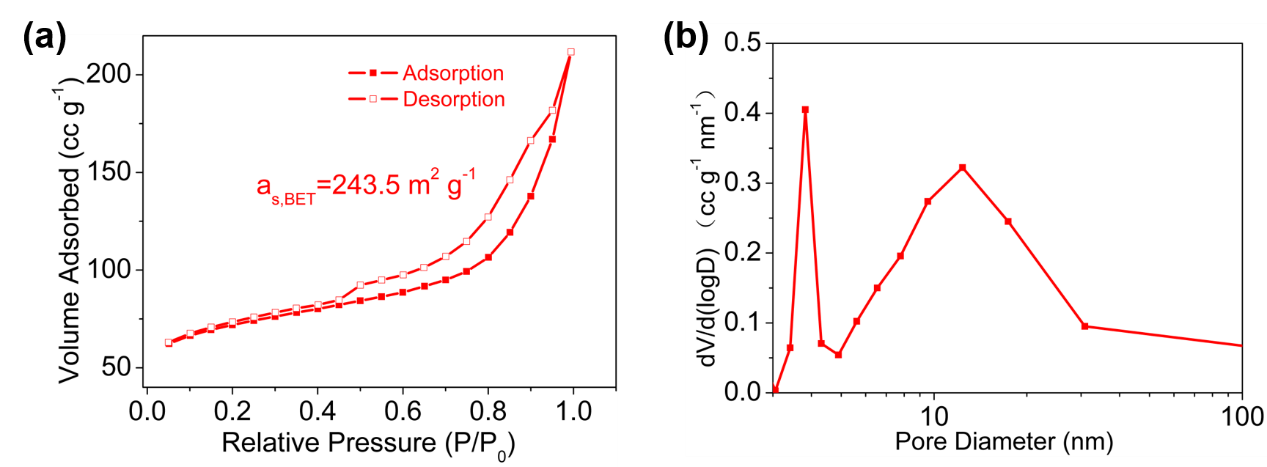
3.1.4 BET测试 14
3.2 电化学表征 15
3.2.1 循环伏安测试 15
3.2.3 首次充放电性能测试 16
3.2.4 循环性能测试 17
3.2.5 倍率性能测试 18
第四章 总结与展望 20
参考文献 22
致 谢 25
第一章 绪论
1.1 引言
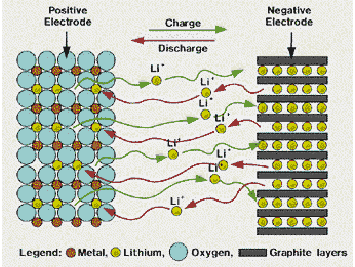
自工业革命以来,人们的日常生活越来越依赖于能源的消耗,解决能源问题成为当今世界发展的关键问题。现代社会,人类的数目呈指数激长,人类对资源的数量和质量的需求也越来越多,但不可再生资源的数目有限。在当代中国,人们仍然以使用不可再生能源为主,这其中,煤、石油和天然气的使用最为频繁,但其燃烧会产生大量含硫、氮和碳的有毒有害气体,对生态环境造成威胁,比如酸雨、两极积雪融化造成的海平面上升和温室效应等。针对此情况,人们研究了风能、太阳能、潮汐能和生物质能等环境友好的清洁能源,但由于间歇性的特点,该能源不能很好的应用于工业和生活。50年代,出现了锂电池[1],70年代中期,锂原电池商业化,90年代,锂离子电池商业化[2],由此,锂离子电池成为最有效、最清洁以及应用最广泛的新能源。锂离子电池的优点有很多,它对环境友好,这就使得环境压力减小;它的寿命长,可多次循环使用。它是手机、电脑、摄像机和电动车等产品的储能设备,更是大型电网和大型储能设备的最佳电动能源[3-4]。
1.2 锂离子电池原理
1.2.1 锂离子电池简介
锂离子电池可以按照其材料类型、电解质以及电池外形类型分类。图1-1为各种外形的锂离子电池。



相关图片展示:
课题毕业论文、开题报告、任务书、外文翻译、程序设计、图纸设计等资料可联系客服协助查找。



