Co-Pi修饰的氧掺杂氮化碳纳米片的制备与光催化性能研究毕业论文
2020-07-05 17:21:45
摘 要
如今,日益严重的环境污染问题和能源资源短缺问题已经引起了人们的关注,半导体光催化技术可以将太阳能代替化石能源利用起来,因此引起了人们的广泛研究。g-C3N4作为一种易合成的新型的非金属材料进入了人们的视野。g-C3N4有相对较小的禁带宽度,可以吸收更多的太阳光中的可见光,它有合适的能带结构,且物理化学性质稳定,无毒无害。目前,g-C3N4已在光解水制氢,CO2光还原及能源转换,污染有机物降解,生物成像等领域进行了广泛探究。然而,纯的g-C3N4可见光响应范围窄,载流子复合率高,电导率低,所以我们采用金属和非金属掺杂、表面光敏化、半导体复合、金属沉积、形貌控制等一系列方法对其改性。其中,离子掺杂是在催化剂中掺杂金属或非金属元素,可以改变g-C3N4的光学性质,电子结构及各种物理化学性质,提高其光催化性能。而非金属掺杂是g-C3N4与掺杂元素价带杂化,提升了价带顶,使其禁带宽度缩小,提高了g-C3N4的光催化活性。然而,掺杂后的催化剂载流子复合率依旧很高,所以我们采用金属沉积的方法。金属沉积是将金属沉积到半导体催化剂表面,金属与半导体之间可以形成空间电荷分离区(称为肖特基势垒)。电子飞速地进行着迁移,使其能够在两种材料的界面处形成了费米能级的平衡。这使得接触中加速了g-C3N4光激发电子的捕获及储存,因此降低了载流子复合率。
本文采用O掺杂,Co-Pi沉积的改性方法,制备光响应范围大,光生载流子复合率低的g-C3N4光催化剂。此外,我们用光催化降解RhB来评估所制备的样品的光催化性能。
主要工作如下:
我们以三聚氰胺,氯化铵和环状糊精为前驱体,采用高温热解法制得氧掺杂的g-C3N4纳米片,随后,我们使用磷酸二氢钠和六水合硝酸钴为原料通过光沉积法进一步制备Co-Pi修饰的氧掺杂氮化碳纳米片。并通过一系列表征手段对其结构和性质做了研究。以降解RhB实验来评估所制备的不同光沉积时间的Co-Pi/OCNS样品的光催化性能。实验结果表明,当光沉积时间为10分钟时,催化剂对RhB的降解效率最高,在70min内的降解率可达到95%。
关键词:氮化碳 掺杂 光沉积 光催化 光降解
ABSTRACT
Nowadays, the problem of increasingly serious environmental pollution and shortage of energy resources has aroused people's attention. Semiconductor photocatalytic technology can use solar energy instead of fossil energy, which has caused people's extensive research. g-C3N4 has entered the field of vision as a new type of non-metallic material that is easy to synthesize. g-C3N4 has a relatively small band gap and can absorb more visible light in sunlight. It has a suitable energy band structure and is stable in physical and chemical properties. It is non-toxic and harmless. At present, g-C3N4 has been extensively explored in the fields of photocatalytic water production, CO2 photoreduction and energy conversion, degradation of organic pollutants, and bioimaging. However, pure g-C3N4 has a narrow range of visible light response, high carrier recombination rate, low conductivity, low quantum efficiency, and small specific surface area. Therefore, we use metal and non-metal doping, surface photosensitization, semiconductor composites, and metal. A series of methods such as deposition and morphology control were modified. Among them, metal and non-metal doping doped with metal or non-metal elements in the catalyst can change the optical properties, electronic structure and physical and chemical properties of g-C3N4. Improve its photocatalytic properties. Non-metal doping is the valence band hybridization of g-C3N4 and doping elements, which enhances the valence band topography, reduces the band gap, and improves the photocatalytic activity of g-C3N4. However, the doping rate of catalyst carriers remains high, so we use metal deposition methods. Metal deposition deposits metal on the surface of a semiconductor catalyst, and a space charge separation region (called a Schottky barrier) can be formed between the metal and the semiconductor. The electrons are migrating so fast that they can form a Fermi level balance at the interface between the two materials. This accelerates the capture and storage of the photo-excited electrons of g-C3N4 during contact, thus reducing the carrier recombination rate.
In this paper, the modified method of O-doping and Co-Pi deposition was used to prepare g-C3N4 photocatalyst with a wide range of photoresponse and low photo-carrier recombination rate. In addition, we used photocatalytic degradation of RhB to evaluate the photocatalytic properties of the prepared samples.
The main work is as follows:
We used melamine, ammonium chloride, and cyclodextrin as precursors to prepare oxygen-doped g-C3N4 nanosheets by high-temperature pyrolysis. Subsequently, we used sodium dihydrogen phosphate and cobalt nitrate hexahydrate to further pass photodeposition. Co-Pi modified oxygen-doped carbon nitride nanosheets were prepared. A series of characterization methods were used to study its structure and properties. The photocatalytic performance of the prepared samples with different Co-Pi concentrations was evaluated by degradation of RhB.The experimental results show that, when the deposition time of Co-Pi is 10 minutes, the efficiency of photodegradation of RhB can reach 95%. Finally, a preliminary study of its reaction mechanism was conducted.
KEYWORDS: C3N4;doped;Photodeposition;photocatalytic;photodegradation
目录
摘 要 I
ABSTRACT i
第1章 绪论 1
1.1 引言 1
1.2光催化的概述 1
1.2.1光催化的原理 1
1.2.2光催化研究背景 2
1.3 石墨相氮化碳的概述 3
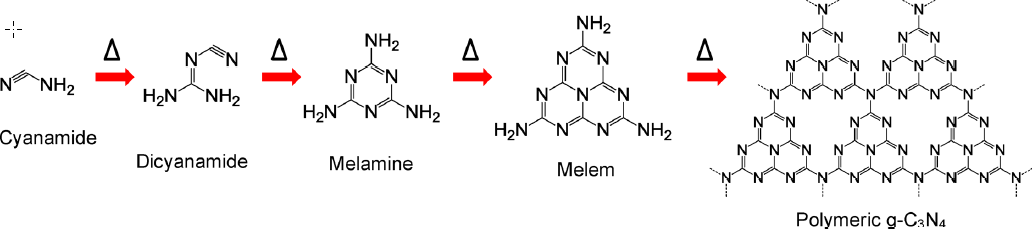
1.3.1 石墨相氮化碳的研究历程 3
1.3.2 石墨相氮化碳的结构与性质 4
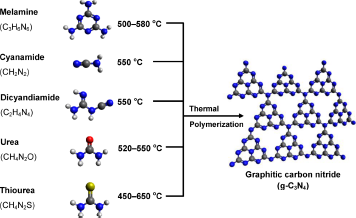
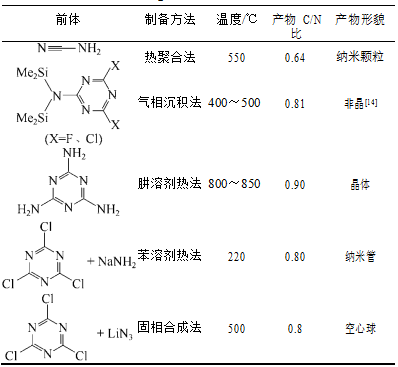
1.3.3石墨相氮化碳的制备方法 6
1.4石墨相氮化碳的改性研究 8
1.4.1 元素掺杂 8
1.4.2金属沉积 9
1.4.3形貌调控 10
1.4.4半导体复合 11
1.4.5光敏化 12
1.5石墨相氮化碳的光催化应用 12
1.5.1光解水制氢气 12
1.5.2 CO2光还原 13
1.5.3光催化有机合成 14
1.6 选题背景及创新点 14
第2章 Co-Pi掺杂的OCNS的制备及其光催化性能的研究 15
2.1引言 16
2.2实验部分 16
2.2.1实验试剂 16
2.2.2催化剂的制备 17
2.2.3催化剂的表征实验 18
2.2.4光催化活性的评估 19
2.3结果与讨论 19
2.3.1表征与结果 19
2.3.2光降解性能 23
2.3.3本章小结 24
参考文献 25
致 谢 30
绪论
1.1 引言
近年来,人们已经逐渐意识到环境污染和能源短缺正在越来越阻碍社会发展同时威胁着人类健康。人类一直使用的都是各种天然的化石能源。但是由于工业化发展迅速和人口的快速增加,化石能源的再生已经逐渐满足不了人类的需求,且化石能源的使用会产生污染。研究者们试图找到一种新的能源材料来代替化石能源以解决能源短缺和环境污染问题,如风能、太阳能等清洁能源。半导体光催化技术由于其安全、经济、可再生等优点[1],是以引起了人们的普遍研究,人们期望它可以代替化石能源。半导体光催化技术是将太阳能通过合适的半导体光催化材料转变成我们可以直接利用的化学能,然后便可进行各种应用诸如光解水以产生H2和O2以及降解有机污染物等。
1.2光催化的概述
1.2.1光催化的原理
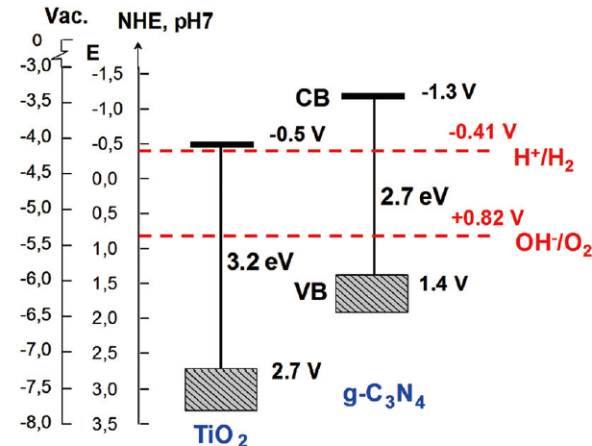
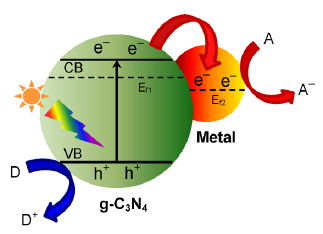
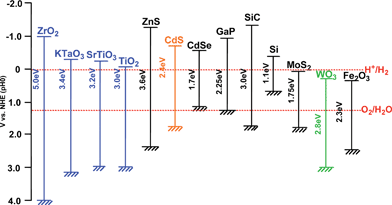
半导体的导电性介于导体和绝缘体之间,它可以在一定条件下变成绝缘体或者导体[2]。根据固体能带理论,半导体的能带不连续,这种特殊结构使半导体具有缺失电子的导带(CB),充满电子的价带(VB)和它们之间的禁带(Band Gap)。半导体的禁带宽度(Eg)是指价带导带之差,一般是1~4 eV,半导体的禁带宽度会影响其光学与电学性质,其禁带宽度值与吸收带波长λg(nm)的关系为 :λg(nm) = 1240/Eg(eV)[3]。我们可以看出,禁带宽度与波长之间是成反比的,其禁带宽度越大, 吸收带波长就越小,光催化性能就弱。
相关图片展示:
课题毕业论文、开题报告、任务书、外文翻译、程序设计、图纸设计等资料可联系客服协助查找。



