还原糖低能耗还原制备Pdγ-Al2O3催化剂及其蒽醌加氢性能毕业论文
2021-02-28 21:44:19
摘 要
H2O2作为一种绿色化工原料被广泛应用于各个领域。蒽醌法为工业生产H2O2的主要方法,该方法的关键步骤为蒽醌类物质催化加氢反应。Pd系催化剂被广泛的应用于蒽醌法制H2O2工艺蒽醌加氢过程。目前催化剂活性组分Pd的传统还原方式能耗大、污染严重,不符合节能减排的要求;利用绿色植物提取液还原催化剂活性组分制备的催化剂虽毋需焙烧,降低了制备能耗,但是其制备工艺繁琐,不利于工业化生产。本文以还原糖(葡萄糖,果糖,麦芽糖)为还原剂,水浴还原分布在载体表层的活性组分Pd制备Pd/γ-Al2O3催化剂,并用于蒽醌加氢过程。采用XRD、TEM、N2吸附-脱附、FT-IR、XPS、AAS和HPLC等表征与测试手段对催化剂的物理化学性质和催化性能进行分析。结果表明,蒽醌加氢30min时,葡萄糖水浴还原制备的催化剂0.4%Pd/γ-Al2O3(G-6.5-55)和传统等体积浸渍法制备的催化剂0.4%Pd/γ-Al2O3的氢化效率相当,且皆低于果糖和麦芽糖水浴还原制备的催化剂;蒽醌加氢反应前期,和葡萄糖水浴还原制备的催化剂相比,活性组分Pd经由果糖和麦芽糖为还原剂制备的催化剂的氢化效率快速上升,蒽醌加氢反应至90min时,其氢化效率即达到最大值;和葡萄糖水浴还原制备的催化剂相比,活性组分Pd经由果糖和麦芽糖为还原剂制备的催化剂氢化效率达到最大值后快速下降,其催化剂稳定性较差;和传统还原方式制备的Pd/γ-Al2O3催化剂相比,上述还原糖还原制备的催化剂的氢化效率和蒽醌循环回收率都有较大提升,还原糖还原制备的典型催化剂0.4%Pd/γ-Al2O3(G-6.5-55)的最高氢化效率达8.0g/L,最高蒽醌循环回收率达92%;和传统等体积浸渍法制备Pd催化剂需要焙烧和催化剂评价时需要H2还原的过程相比,使用还原糖水浴还原分布在载体表层的活性组分Pd毋需上述步骤,因而简化了催化剂的制备工艺,降低了能耗,并提高了催化剂的制备效率。
关键词:H2O2; 蒽醌法; 加氢; Pd; 还原糖
Abstract
As a green chemical raw material, H2O2 is widely used in various fields. The anthraquinone process is the main method for the industrial production of H2O2. The key step of the method is the catalytic hydrogenation of anthraquinone. Pd-based catalysts have been extensively utilized in anthraquinone hydrogenation during the anthraquinone method of H2O2 production process at present. The traditional way to reduce the catalyst active component Pd has large energy consumption and serious pollution, which can’t meet the requirements of energy saving and emission reduction. Although the catalysts prepared by the green plant extract reducing catalyst active component do not need to be calcined and the preparation of energy consumption is reduced, tedious process is not conducive to industrial production. In this paper, we use reducing sugar (glucose, fructose, maltose) as reducing agent and reduce in water bath catalyst active component Pd on the surface of the carrier to prepare Pd/γ-Al2O3 catalyst. The catalysts were evaluated in anthraquinone hydrogenation. Physical chemistry properties and catalytic performance of the catalysts were comparatively studied by XRD, TEM, N2 adsorption-desorption, FT-IR, XPS, AAS and HPLC. The results show that, when anthraquinone is hydrogenated for 30 min, the hydrogenation efficiency of the catalyst prepared by glucose reducing in water bath (0.4%Pd/γ-Al2O3(G-6.5-55)) and the catalyst prepared by conventional impregnation method (0.4%Pd/γ-Al2O3) was approximate, but both are lower than the catalysis prepared by fructose and maltose reducing in water bath. At the early stage of anthraquinone hydrogenation, the hydrogenation efficiency of the catalysts prepared by the fructose and maltose reducing in water bath increased faster than the catalyst prepared by glucose reducing in water bath. When anthraquinone is hydrogenated for 90 min, the hydrogenation efficiency of the catalysts prepared by the fructose and maltose reducing in water bath reached the maximum. In comparison with the catalyst prepared by glucose reducing in water bath, the hydrogenation efficiency of the active component Pd reduced by fructose and maltose decreased rapidly after reaching the maximum value. So the stability of the catalyst prepared by fructose and maltose reducing in water bath is poorer. The hydrogenation efficiency and the anthraquinone recycling rate of the catalyst prepared by the reducing sugar reducing was greatly improved compared with those of the Pd/γ-Al2O3 catalyst prepared by the traditional impregnation method. The typical catalysts (0.4%Pd/γ-Al2O3(G-6.5-55)) prepared by the reducing sugar method, its hydrogenation efficiency can reach up to 8.0g/L, and anthraquinone recycling rate can reach up to 92%. Compared with isometric impregnation processes to preparation Pd catalysts, which need calcination and catalyst evaluation requiring H2 reduction, using reducing sugar (glucose, fructose, maltose) as reducing agent and reducing catalyst active component Pd on the surface of the carrier in water bath to prepare Pd/γ-Al2O3 catalyst decreases the above steps, thus greatly simplifies the preparation process, dramatically reduces the energy consumption and significantly improves the preparation efficiency.
Key Words: H2O2; anthraquinone; hydrogenation; Pd; reducing sugar.
目 录
摘 要 I
Abstract II
第1章 绪论 1
1.1. 蒽醌法制H2O2氢化过程Pd系催化剂研究现状 1
1.1.1. 蒽醌法生产H2O2工艺简介 1
1.1.2. 蒽醌加氢制备氢蒽醌Pd系催化剂研究进展 2
1.2. 催化剂活性组分Pd的传统还原方式简介 3
1.3. 绿色合成纳米金属粒子 3
1.3.1. 生物质提取液 3
1.3.2. 生物质有效成分单质 4
1.4. 选题背景及研究内容 4
1.4.1. 本文选题背景 4
1.4.2. 研究内容 5
第2章 实验部分 6
2.1. 实验药品及仪器 6
2.2. 催化剂制备 7
2.2.1. 还原糖还原Pd催化剂的制备 7
2.2.2. 传统Pd/γ-Al2O3催化剂的制备 7
2.3. 催化剂的表征 9
2.3.1. XRD 9
2.3.2. N2吸附-脱附 9
2.3.3. TEM 10
2.3.4. AAS 10
2.3.5. FT-IR 10
2.3.6. XPS 10
2.4. 催化剂性能评价 10
2.4.1. 催化活性 10
2.4.2. 蒽醌循环回收率 12
第3章 结果分析与讨论 13
3.1. 催化剂表征 13
3.1.1. XRD 13
3.1.2. TEM 14
3.1.3. N2吸附-脱附 14
3.1.4. FT-IR 19
3.1.5. XPS 21
3.2. 还原糖-催化剂的相互作用机制 22
3.2.1. 葡萄糖 22
3.2.2. 果糖 23
3.2.3. 麦芽糖 25
3.3. 催化剂性能评价 26
3.3.1. Pd负载量对催化性能的影响 26
3.3.2. 催化剂前驱物-还原糖溶液体系对催化剂性能影响 27
第4章 结论与展望 34
4.1. 结论 34
4.2. 展望 35
参考文献 36
致 谢 41
绪论
蒽醌法制H2O2氢化过程Pd系催化剂研究现状
过氧化氢(H2O2)作为一种不会产生二次污染的绿色化学品,被广泛的应用于各个生产,生活领域[1]。目前生产H2O2的主要方法为蒽醌法,其产能大约占H2O2总产能的99%[2]。
蒽醌法生产H2O2工艺简介
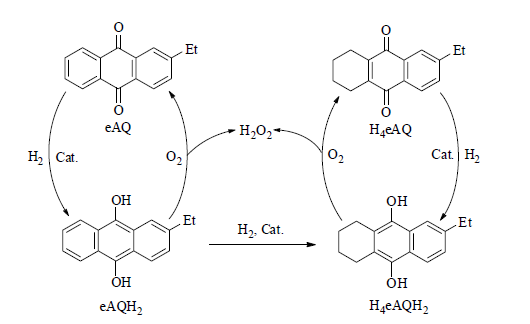
蒽醌法生产H2O2工艺流程主要由蒽醌工作液中有效蒽醌类物质加氢生成氢蒽醌,氢蒽醌氧化,萃取,提纯四个过程构成,其中蒽醌类物质催化加氢反应氢蒽醌是该工艺的关键步骤[3],蒽醌工作液中有效蒽醌类物质为2-乙基蒽醌(eAQ)和四氢-2-乙基蒽醌(H4eAQ),在催化剂作用下蒽醌分子中C=O与氢气发生加成反应,生成二氢-2-乙基蒽醌(eAQH2)和六氢-2-乙基蒽醌(H4eAQH2)。用氧气直接氧化经氢化后的蒽醌工作液,eAQH2和H4eAQH2氧化生成eAQ和H4eAQ循环使用,同时生成H2O2(如图1.1所示)。
课题毕业论文、开题报告、任务书、外文翻译、程序设计、图纸设计等资料可联系客服协助查找。



