两亲性柱芳烃对六价铬的吸附毕业论文
2020-04-15 00:15:09
摘 要
随着全球工业化的进程,铬产品在生产中应用十分广泛,其在各个行业领域中的应用或排放遗留下非常严重的环境危害,由于其生物累积性,容易随食物链富集对人类或其他动物产生中毒。六价铬是铬的高氧化态,其危害性较其他价态铬高得多。目前针对其的污染治理方法也正在不断深入,吸附法是较为常见的一种方法,各方面因素要求制备成本低、使用便捷、适用范围广泛的六价铬吸附材料迫在眉睫。
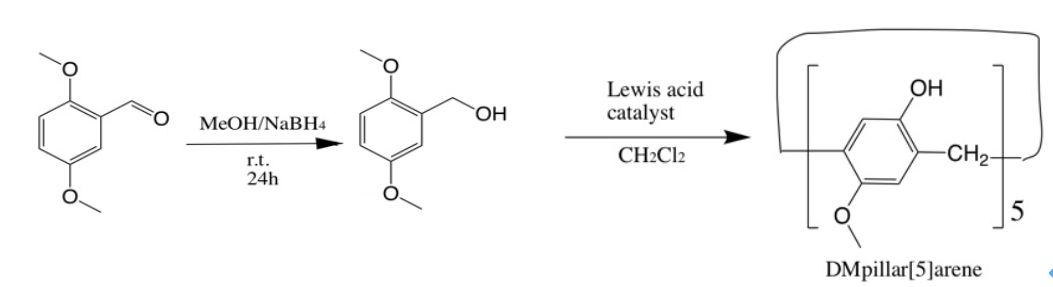
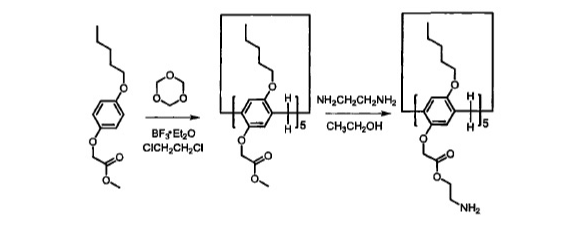
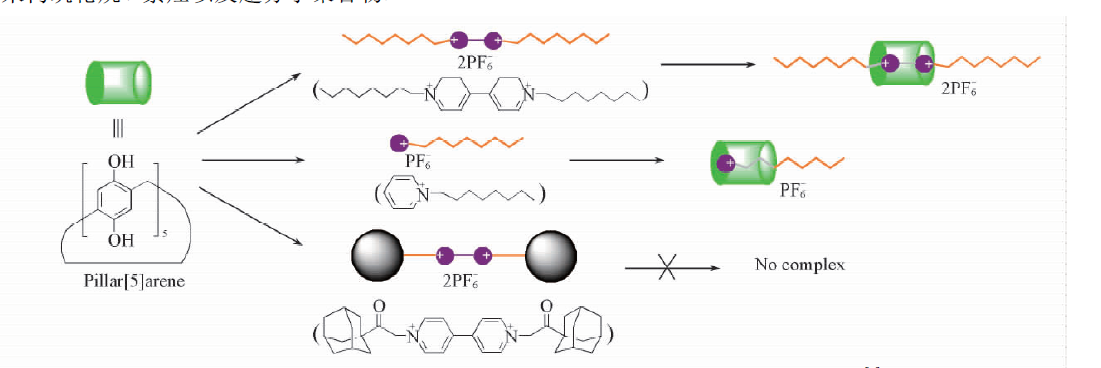
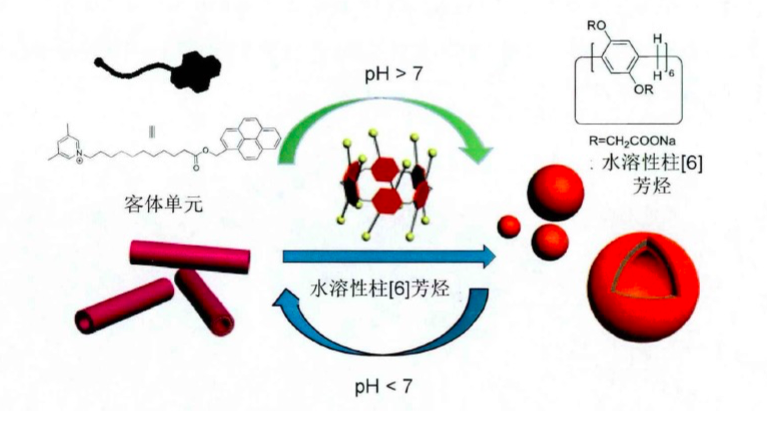
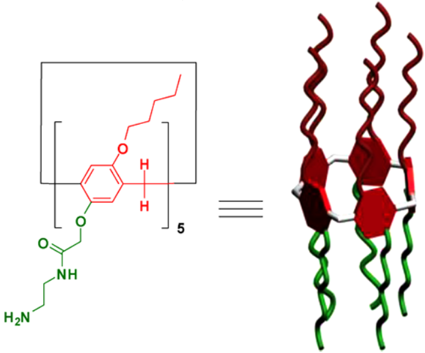
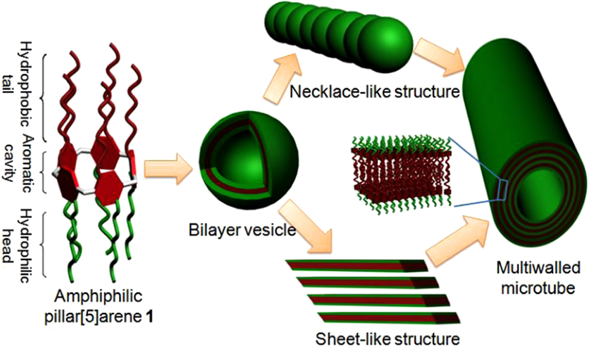
自主客体研究发现冠醚进而形成超分子化学学科以来,已有多代大环主体分子相继问世并展开研究,柱芳烃是十年前新发现的一类内部空腔含密集电子云的柱状大环分子,其柱两端具可修饰性。柱[5]芳烃是柱[n]芳烃系列中环张力最小,空间构型比较稳定的主要产物,本文分别以实验室自制的柱[5]芳烃5a,5b,5c作为吸附剂,探究其对六价铬的吸附性能,选择重铬酸钾(Cr6 )作为实验中吸附对象,利用分光光度计绘制了Cr6 溶液的标准曲线,其浓度与吸光度的线性方程为A=0.70838c 0.00702,线性相关系R2=0.99883。探讨了两亲性柱芳烃烷基链长与pH对吸附性能的影响,优选吸附剂5a作为实验对象,讨论了5a投入量、Cr6 溶液不同初始浓度对吸附性能的影响。实验结果表明,在一定范围内(pH不低于2),5a的吸附性能随着Cr6 溶液pH降低而表现越好,实验中测得最佳pH=4;吸附去除率随着5a投放量的增加而提升,综合取3g/L为最佳投放量;吸附容量随着Cr6 初始浓度增大而逐渐增加,吸附容量最大可达42.89mg/g,但是吸附率随着Cr6 初始浓度增大而逐渐减少。
实验中通过利用准一级、准二级两种吸附动力学方程模型来分别研究了该吸附过程中的动力学。根据研究结果来看,相比之下两种模型后者更加符合研究中吸附剂5a对Cr6 的吸附实际过程,属于物理吸附的类别。并且5a经过5次解吸-吸附循环过程,仍保持着较高的吸附性能。
关键词:柱芳烃 主客体化学 Cr6 吸附
Adsorption of Cr6 by Amphiphilic Pillararenes
Abstract
With the global industrialization process, chromium products are widely used in production, and their application or discharge in various industrial fields,which left a very serious environmental hazard. Due to their bioaccumulation, it is easy to enrich Hexavalent chromium on the human or other animals to be poisoned with the food chain. Hexavalent chromium is a highly oxidized state of chromium, which is much more harmful than other valence chromium. At present, the pollution control methods for them are also in-depth, and the adsorption method is a relatively common method. All the factors require that the hexavalent chromium adsorption material with low preparation cost, convenient use and wide application range is imminent.
Since the formation of crown ethers from the study of Host-guest property, and the development of supramolecular chemistry, many generations of macrocyclic host molecules have been successively launched and researched. Pillararenes are kinds of fashionable columnar macrocyclic molecules with dense electron clouds inside cavities. The ends of the pillar are modifiable. The pillar[5] arene is the main product with the smallest ring tension and stable spatial configuration in the pillar [n] arene series. In this research, the pillar[5]arene 5a, 5b, 5c(made by self-laboratory) is applied as the adsorbent for exploring the adsorption property of Cr6 . potassium dichromate (Cr6 ) was selected as the adsorption target in the experiment. Spectrophotometer was employed to collected data for drawing standard curve of Cr6 solution. The linear equation of concentration and absorbance was A=0.70838c 0.00702, linear relationship R2 = 0.99883. The effect of alkyl chain length and pH on the adsorption performance of amphiphilic pillararenes was discussed. The adsorbent 5a was selected as the experimental object. The different input of adsorbent-5a and varied initial concentrations of Cr6 solution were discussed about the adsorption property. The experimental results are presented, in a certain range(pHgt;2), the adsorption property of 5a gets better with the lower pH of Cr6 solution. The optimum pH is 4 in the experiment. The adsorption rate increases as input of 5a does. The best is the 3g/L. The amount of adsorption got increased as the increase of initial concentration of Cr6 , and the best adsorption amount was 42.89 mg/g, but the adsorption rate decreased while the initial concentration of Cr6 has grown.
The kinetics of the adsorption process was studied by quasi-first-order kinetic equations and quasi-second-order kinetic equations. According to the results, the 5a’s adsorption process of Cr6 is more like the quasi-secondary adsorption kinetics model, the whole adsorption process follows physical adsorption. And 5a has maintained high adsorption performance after 5 desorption-adsorption cycles.
Key words: Amphiphilic pillararenes ;Host-guest chemistry; Cr6 ; adsorption
以上是毕业论文大纲或资料介绍,该课题完整毕业论文、开题报告、任务书、程序设计、图纸设计等资料请添加微信获取,微信号:bysjorg。
相关图片展示:
课题毕业论文、开题报告、任务书、外文翻译、程序设计、图纸设计等资料可联系客服协助查找。



