1,2,4-丁三醇转化液的回收研究毕业论文
2020-04-15 20:30:29
摘 要
1,2,4-丁三醇被广泛应用于军事、医药、烟草、高分子材料等领域。本文采用木糖-丁三醇转化法,以1,2,4-丁三醇的发酵液为原料,通过静态吸附法筛选到疏水性微孔吸附树脂HD-6。利用动态吸附实验,研究发酵液在HD-6树脂上的吸附-洗脱情况,发现发酵液穿透洗脱曲线中存在增浓效果。经初步判断:发酵液中的盐对树脂吸附1,2,4-丁三醇产生促进作用。之后采用静态吸附法,系统的研究了Na2HPO4、KH2PO4、NaCl三种盐以及阳离子和阴离子对树脂吸附1,2,4-丁三醇的影响;利用动态法考察了单组分Na2HPO4和三种混合盐对树脂吸附1,2,4-丁三醇的影响;还考察了流速、不同配比的混合盐对树脂吸附的影响。
结果表明,120 g/L的Na2HPO4对树脂吸附1,2,4-丁三醇的促进作用最好,其中在考察离子对树脂吸附影响时发现:阳离子半径越小,盐促效果越好;阴离子的电荷荷数越大,盐促效果越好。在动态实验中,流速为1 min/L时单组分和多组分盐对促进树脂吸附1,2,4-丁三醇的效果更佳,同时盐和1,2,4-丁三醇也有明显的分离且回收率可达到97%以上。对比多组分混合盐实验发现:Na2HPO4浓度为0.1 mol/L,KH2PO4浓度为0.30 mol/L,NaCl的浓度为0.20 mol/L时,盐促效果更明显,更有利于树脂吸附1,2,4-丁三醇。
关键词:1,2,4-丁三醇 树脂吸附 盐效应 色谱分离
Effect of three inorganic salts on the adsorption properties of
1,2,4-butanetriol on resin
Abstract
1,2,4-butanetriol is widely used in military, pharmaceutical, tobacco, polymer materials and other fields.The article adopts xylose-butantriol conversion method.Using 1,2,4-butanetriol fermentation broth as raw material, and the hydrophobic microporous adsorption resin HD-6 was screened by static adsorption method.Dynamic adsorption experiment was made for studying the adsorption-elution of the fermentation broth on HD-6 resin, it was found that there was a thickening effect in the breakthrough curve of the fermentation broth.It was preliminarily judged that the salt in the fermentation broth promoted the adsorption of 1,2,4-butantriol on the resin. Then, using static adsorption method to research the effects of three salts of Na2HPO4, KH2PO4 and NaCl, and the cations and anions on the adsorption of 1,2,4-butantriol on the resin.The effects of one-component Na2HPO4 and three mixed salts on the adsorption of 1,2,4-butantriol on the resin were investigated by dynamic method. The effects of flow rate and mixed ratios of different ratios on resin adsorption were also investigated.
The results showed that 120 g/L Na2HPO4 promoted the adsorption of 1,2,4-butantriol to the resin.When investigating the effect of ions on the adsorption of resin, it is found that the smaller the cation radius, the better the salt promoting effect; the larger the charge charge of the anion, the better the salt promoting effect.In the dynamic experiment, the single-component and multi-component salts have better effect on promoting the adsorption of 1,2,4-butantriol by the resin at a flow rate of 1 min/L.At the same time, salt and 1,2,4-butantriol also have obvious separation and the recovery rate can reach over 97%.Comparison of multi-component mixed salt experiments, it was found that the concentration of Na2HPO4 was 0.10 mol/L, the concentration of KH2PO4 was 0.30 mol/L, and the concentration of NaCl was 0.20 mol/L, the effect of salt promotion was more obvious, which was more favorable for the adsorption of 1,2,4-butantriol by resin.
Key words: 1,2,4-butanetriol; resin adsorption; salt effect; chromatographic separation
目录
摘要 I
ABSTRACT II
第一章 文献综述 1
1.1前言 1
1.2 丁三醇简介 1
1.2.1 丁三醇的理化性质 1
1.2.2丁三醇工业生产用途 1
1.3丁三醇合成方法 2
1.3.1化学合成 2
1.3.2生物合成 2
1.4生物基多元醇分离纯化技术研究进展 4
1.4.1发酵液的预处理 4
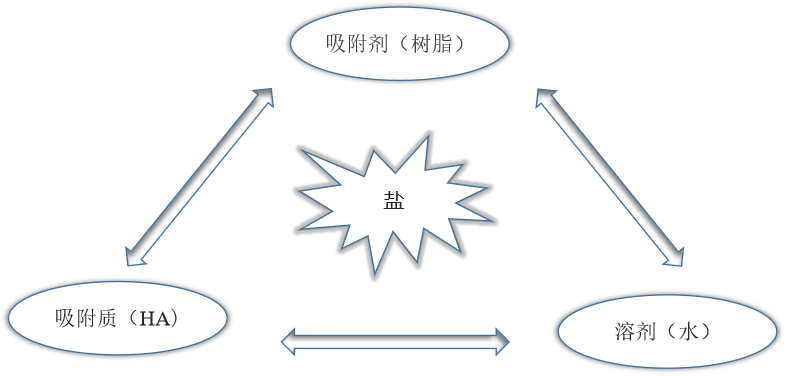
1.4.2盐的去除 5
1.4.3目标产物的浓缩纯化 6
1.5本论文选题目的及研究内容 7
1.5.1选题目的 7
1.5.2研究内容 7
第二章 树脂筛选和静态吸附研究 9
2.1引言 9
2.2实验部分 9
2.2.1实验仪器与试剂 9
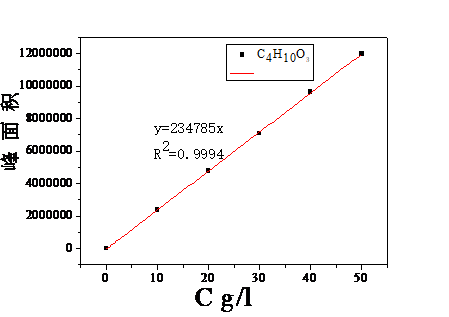
2.2.2绘制标准曲线 10
2.2.3发酵液中化学物质检测 11
2.2.4树脂筛选 12
2.2.5静态吸附实验 13
2.3分析方法 14
2.4结果与讨论 15
2.4.1树脂初筛 15
2.4.2发酵液上柱实验检测 16
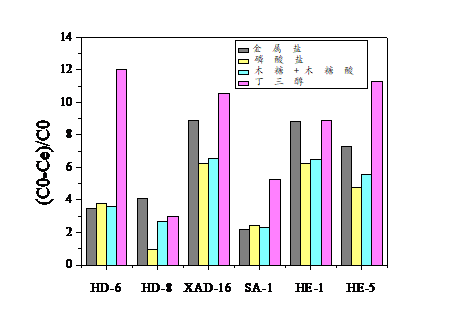
2.4.3不同盐对丁三醇吸附量的影响 18
2.4.4盐Na2HPO4的最佳浓度 20
2.4本章小结 21
第三章 单柱动态吸附研究 22
3.1引言 22
3.2实验部分 22
3.2.1实验仪器与试剂 22
3.2.2单组分和多组分盐上柱实验 22
3.2.3流速对上柱实验的影响 23
3.2.4不同投料量对多组分盐上柱实验的影响 23
3.2.5洗脱液的选择 23
3.3结果与讨论 23
3.3.1单组分盐和多组分盐对吸附影响 23
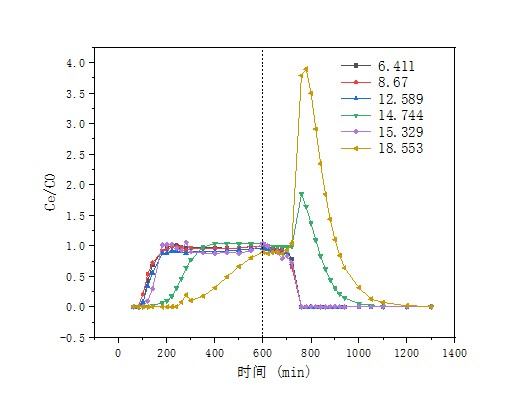
3.3.2 不同流速对动态吸附实验影响 25
3.3.3不同配比混合盐对树脂吸附的影响 27
3.4本章小结 28
第四章 结论与展望 30
4.1结论 30
4.2展望 30
参考文献 31
致谢 34
第一章 文献综述
1.1前言
近年来,全球面临着工业化持续发展而自然资源相对匮乏的矛盾,利用生物基产品已经成为当前研究的关注点[1]。生物基产品一般采用可再生的生物质资源代替化工原料,同时具有绿色低碳、耗时少、成本低且污染少的优点[2]。目前,各国政府都认识到了生物基产品在有效利用生物质资源、节能减排和保护环境方面的作用,并都采取了一定措施发展生物基产品[1]。据USDA研究报告,生物基化学品在未来几年后将占据全球化学品22%的市场地位,产值超过5000亿美元/年[3]。当前,研究的生物基产品有:乳酸、丙二醇、1,4-丁二醇、脂肪醇、甘油、1,3-丙二醇、对二甲苯、1,2,4-丁三醇等,这些生物平台化合物广泛应用于各个行业。但目前利用生物合成方法得到的生物基产品的产率都较低,故寻求一种高效的分离提纯方法是目前生产的关键。
1.2 丁三醇简介
1.2.1 丁三醇的理化性质
1,2,4-丁三醇(BT)是一种无色无味、透明的粘稠状液体。分子式为C4H10O3,分子量为106,CAS号为3068-00-6。从结构式可看出1,2,4丁三醇有三个亲水性羟基,因此在水和醇类物质中溶解度较高,并且它的第二个C是手性碳,具有旋光性[4]。1,2,4-丁三醇主要理化性质见表1.1。
以上是毕业论文大纲或资料介绍,该课题完整毕业论文、开题报告、任务书、程序设计、图纸设计等资料请添加微信获取,微信号:bysjorg。
相关图片展示:

课题毕业论文、开题报告、任务书、外文翻译、程序设计、图纸设计等资料可联系客服协助查找。



